Resources for clinical innovation
Your source of practical guides, expert webinars, interesting articles, industry events and opinion pieces to accelerate digital endpoint research and collaboration
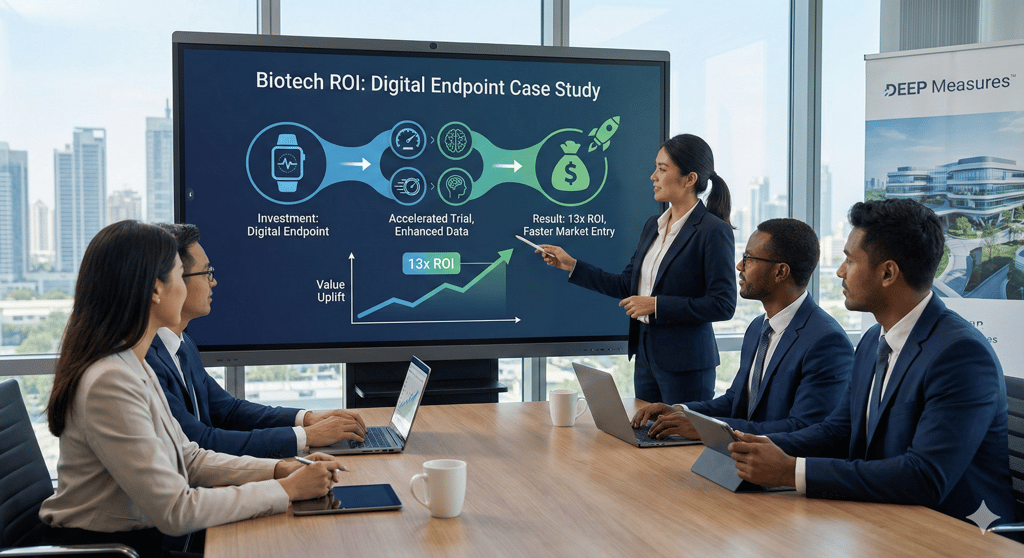
What if a single digital endpoint could change the trajectory of an entire clinical program? Faced with a small Phase 2 study and endpoints not able to show true therapeutic impact, a biotech turned...
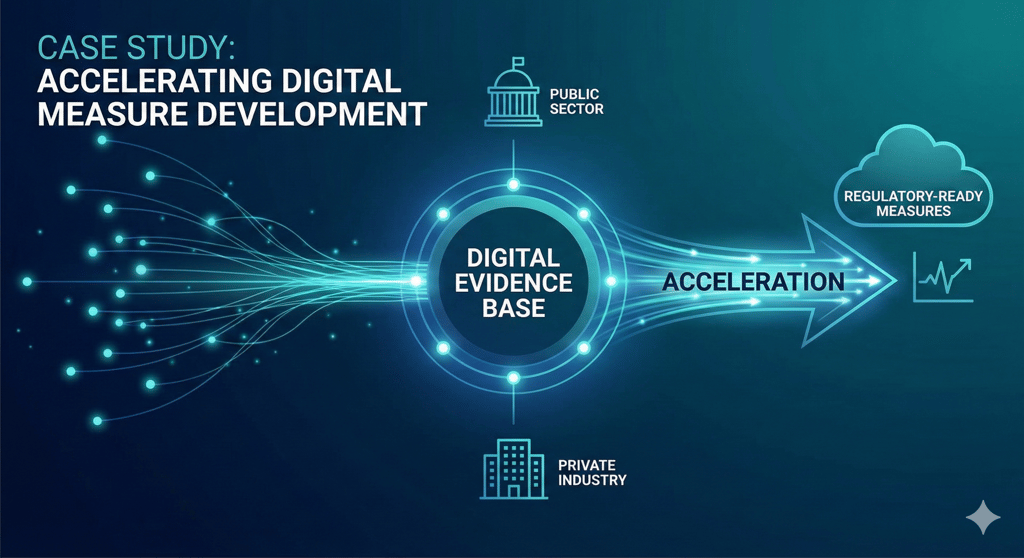
Pharma has spent years generating evidence for digital measures - yet much of it sits unused, scattered across public‑private partnerships and legacy research. What if the key to accelerating...