Resources for clinical innovation
Your source of practical guides, expert webinars, interesting articles, industry events and opinion pieces to accelerate digital endpoint research and collaboration
Posts by Team DEEP Measures
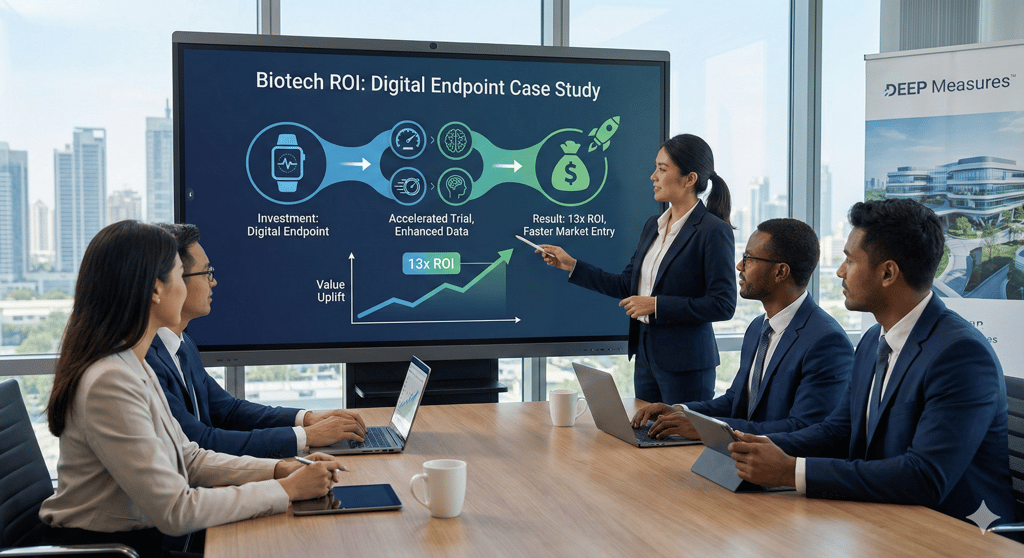
What if a single digital endpoint could change the trajectory of an entire clinical program? Faced with a small Phase 2 study and endpoints not able to show true therapeutic impact, a biotech turned...
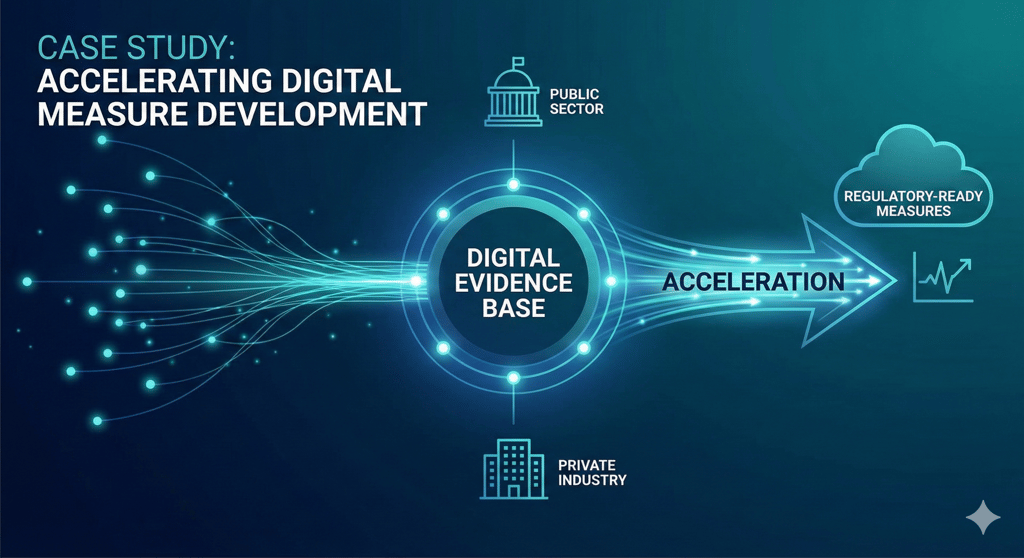
Pharma has spent years generating evidence for digital measures - yet much of it sits unused, scattered across public‑private partnerships and legacy research. What if the key to accelerating...
We rarely think about what makes routine health checks (like a simple blood‑pressure reading) so effortless. Clear definitions, shared standards and trusted tools make it possible. Digital measures...
A new, contactless way to measure sleep and OSA is reshaping metabolic trials. Pharma teams can finally capture high‑quality OSA endpoints without wires, clinics, or patient burden. An ideal read for...